Calculations Involving Moles
Chemists aren't usually concerned with how many hot dogs they can buy on a given day. They would likely be concerned about reactions that they are going to perform.
Consider the following reaction:
Chemists aren't usually concerned with how many hot dogs they can buy on a given day. They would likely be concerned about reactions that they are going to perform.
Consider the following reaction:
2 H2 + O2 ==> 2 H2O
Suppose a chemist wanted to perform this reaction to produce water. There would be several things the chemist would need to consider. Among the considerations:
For this particular problem, a chemist needs to produce 40.0 mol H2O. How many mol H2 must the chemist react?
Step 1: Write what you are given.
In this problem, the given is 40.0 mol H2O.
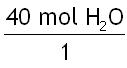
Step 2: Pick the correct mole ratio conversion factor.
The following are all of the possible mole ratios:
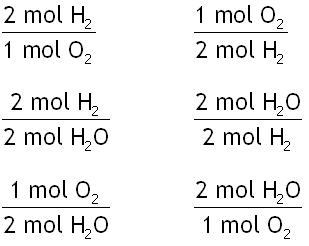
You only need one of them, so which one do you pick? Note the following:

Step 3: Cancel the units.
Cancel the same units that appear on the top and bottom.
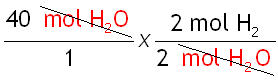
Step 4: Do the math.
Remember to multiply by #'s on the top and divide by #'s on the bottom.
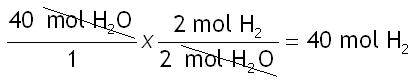
The answer should be reported as 40.0 mol H2. The given in this problem (40.0 mol H2O) had 3 sig figs, therefore the answer should have 3 sig figs as well.
Before clicking "NEXT" to go on to the next page, see if you can do the following problem on paper. Then click "NEXT" and it will be explained for you on the next page.
Using the reaction:
2 H2 + O2 ==> 2 H2O
Determine how many mol O2 are needed to react with 36 mol H2.
- How much H2 is needed
- How much H2O will be produced
- Safety concerns: This reaction gives off a lot of energy!
For this particular problem, a chemist needs to produce 40.0 mol H2O. How many mol H2 must the chemist react?
Step 1: Write what you are given.
In this problem, the given is 40.0 mol H2O.
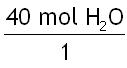
Step 2: Pick the correct mole ratio conversion factor.
The following are all of the possible mole ratios:

You only need one of them, so which one do you pick? Note the following:
- The given in the problem is "40 mol H2O"
- The unknown in the problem is "mol of H2"

Step 3: Cancel the units.
Cancel the same units that appear on the top and bottom.
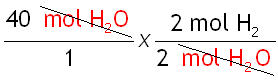
Step 4: Do the math.
Remember to multiply by #'s on the top and divide by #'s on the bottom.
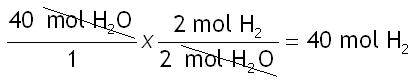
The answer should be reported as 40.0 mol H2. The given in this problem (40.0 mol H2O) had 3 sig figs, therefore the answer should have 3 sig figs as well.
Before clicking "NEXT" to go on to the next page, see if you can do the following problem on paper. Then click "NEXT" and it will be explained for you on the next page.
Using the reaction:
2 H2 + O2 ==> 2 H2O
Determine how many mol O2 are needed to react with 36 mol H2.
Last modified: Tuesday, July 13, 2010, 1:10 PM