The Make-Up of a % Yield Problem
An example problem:
2 H2 + O2 -----> 2 H2O
In the laboratory, 30.0 g of H2 was reacted with excess O2 and 200. g of H2O was produced. What is the % yield of the H2O?
Actual Yield:
The actual yield is usually given in the problem statement (If it is not, then the % yield must be given in the problem statement). Notice that the question asks about the % yield of H2O. That means that the number that relates to the H2O is the actual yield.
200. g of H2O is the actual yield in this case.
Theoretical Yield:
Be careful, the 30.0 g of H2 IS NOT the theoretical yield! You have to use it to get the theoretical yield. You can do a mass - mass problem between H2 and H2O to determine the theoretical yield of H2O (268 g of H2O).
Percent Yield:
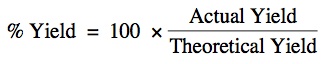
% Yield = 100 x (200/268) = 74.7% Yield
An example problem:
2 H2 + O2 -----> 2 H2O
In the laboratory, 30.0 g of H2 was reacted with excess O2 and 200. g of H2O was produced. What is the % yield of the H2O?
Actual Yield:
The actual yield is usually given in the problem statement (If it is not, then the % yield must be given in the problem statement). Notice that the question asks about the % yield of H2O. That means that the number that relates to the H2O is the actual yield.
200. g of H2O is the actual yield in this case.
Theoretical Yield:
Be careful, the 30.0 g of H2 IS NOT the theoretical yield! You have to use it to get the theoretical yield. You can do a mass - mass problem between H2 and H2O to determine the theoretical yield of H2O (268 g of H2O).
Percent Yield:
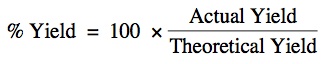
% Yield = 100 x (200/268) = 74.7% Yield
Last modified: Thursday, August 12, 2010, 11:23 AM