Problem:
Using the reaction:
2 H2 + O2 ==> 2 H2O
Determine how many mol O2 are needed to react with 36 mol H2.
Step 1: Write what you are given.
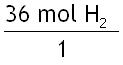
Step 2: Pick the correct mole ratio conversion factor.
Since "H2" and "O2" are both in the problem, it should contain both "H2" and "O2" in the conversion factor. Choose the one with "mol H2" on the bottom to cancel out the "mol H2" on the top.
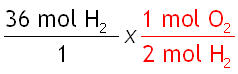
Step 3: Cancel the units.
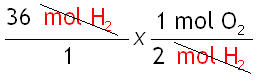
Step 4: Do the math.
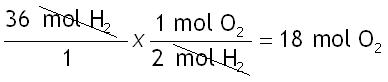
The answer should be reported as 18 mol O2.
There are 2 sig figs in the given (36 mol H2), therefore the answer should also have 2 sig figs.
Using the reaction:
2 H2 + O2 ==> 2 H2O
Determine how many mol O2 are needed to react with 36 mol H2.
Step 1: Write what you are given.
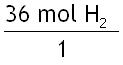
Step 2: Pick the correct mole ratio conversion factor.
Since "H2" and "O2" are both in the problem, it should contain both "H2" and "O2" in the conversion factor. Choose the one with "mol H2" on the bottom to cancel out the "mol H2" on the top.
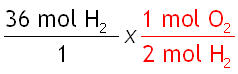
Step 3: Cancel the units.
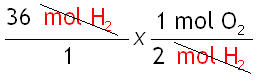
Step 4: Do the math.
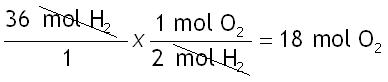
The answer should be reported as 18 mol O2.
There are 2 sig figs in the given (36 mol H2), therefore the answer should also have 2 sig figs.
Last modified: Tuesday, July 13, 2010, 1:11 PM