Where to start and stop on the road map...
Assume you are dealing with the following reaction:
S(s) + 3 F2(g) ==> SF6(g)
(s) stands for solid
(g) stands for gas
You could be asked a number of questions to solve involving the reaction. I'll ask some sample questions involving the above reaction. DO NOT SOLVE THE QUESTIONS!!! Instead, jot down where you would start and where you would stop on the full road map. The answers are listed below the road map on this page. Check your answers when you are done.
MAKE SURE TO FIGURE OUT THE GIVEN & THE UNKNOWN!!!
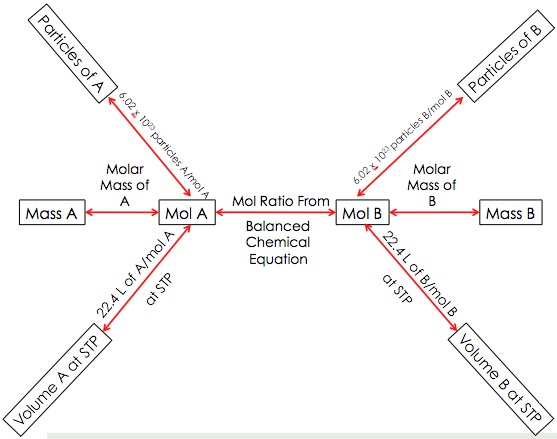
Answers:
Assume you are dealing with the following reaction:
S(s) + 3 F2(g) ==> SF6(g)
(s) stands for solid
(g) stands for gas
You could be asked a number of questions to solve involving the reaction. I'll ask some sample questions involving the above reaction. DO NOT SOLVE THE QUESTIONS!!! Instead, jot down where you would start and where you would stop on the full road map. The answers are listed below the road map on this page. Check your answers when you are done.
MAKE SURE TO FIGURE OUT THE GIVEN & THE UNKNOWN!!!
- How many mol of F2 is needed to react with 10.0 mol of S?
- How many g of S reacts to form 30.0 g of SF6?
- If 20. mol of SF6 are formed, how many grams of S reacted?
- How many mol of F2 reacts with 100. g of S?
- 30.0 g of S reacts to form how many molecules of SF6?
- At STP, how many L of F2 is needed to form 10.0 x 1030 molecules of SF6?
- How many mol of S is needed to form 65.0 L of SF6 at STP?

Answers:
- Start ==> mol A; End ==> mol B
- Start ==> mass A; End ==> mass B
- Start ==> mol A; End ==> mass B
- Start ==> mass A; End ==> mol B
- Start ==> mass A; End ==> Particles B
- Start ==> Particles A; End ==> Volmue B at STP
- Start ==> Volume A at STP; End ==> mol B
Last modified: Tuesday, July 27, 2010, 3:39 PM