N2 + 3 H2 ==> 2 NH3
But what if I do not start with the exact amount of needed reactants?
In this case, assume that I add 5 N2 molecules and 9 H2 molecules to a reaction flask. I could ask the following questions:
The answers to the questions above are:
Limiting and excess reagent problems are among the most challenging in beginning chemistry. Make sure to review problems several times, ask questions, work hard, do not skip steps, and do not get frustrated!!
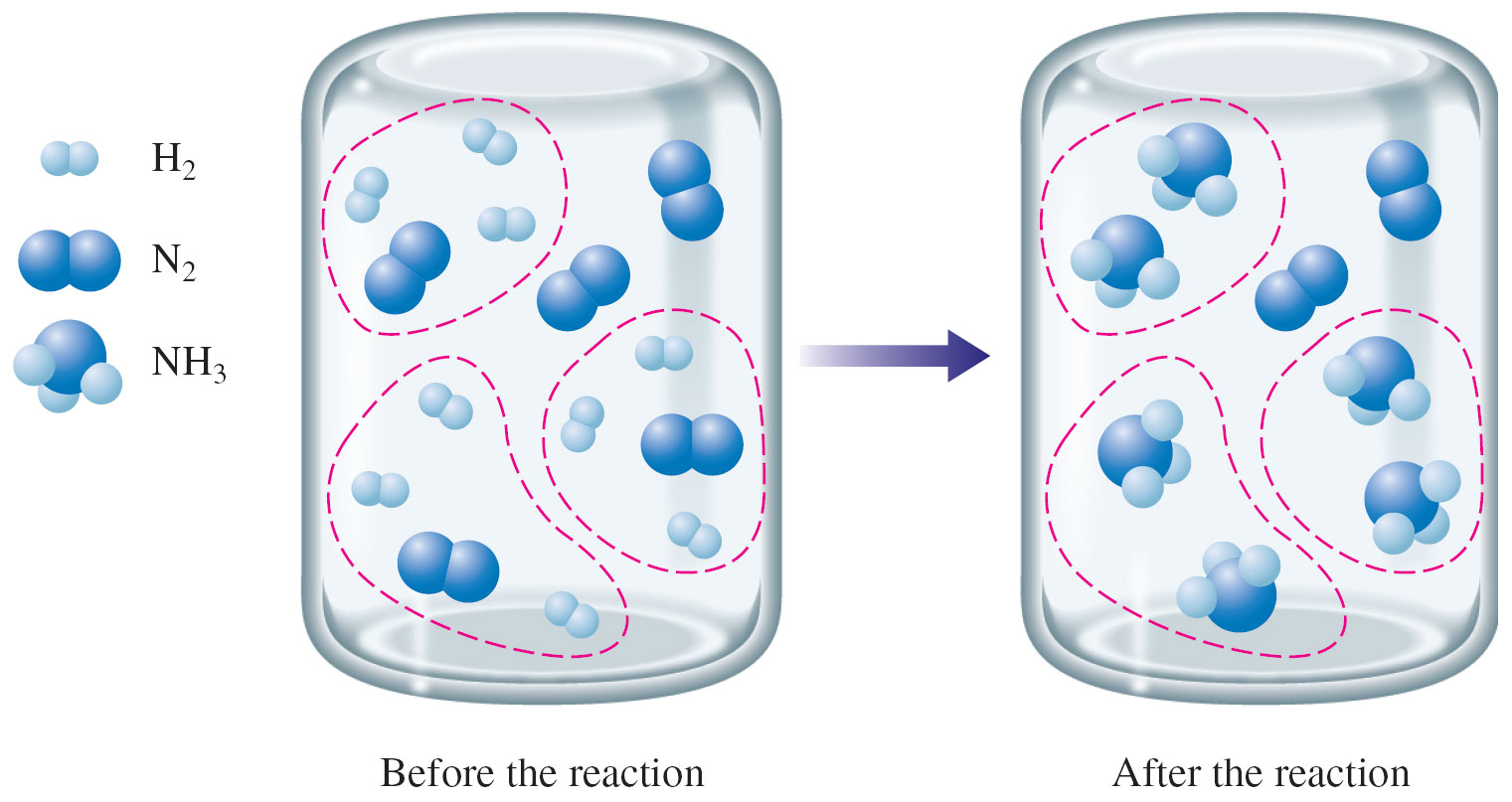
But what if I do not start with the exact amount of needed reactants?
In this case, assume that I add 5 N2 molecules and 9 H2 molecules to a reaction flask. I could ask the following questions:
- Which reagent (N2 or H2) is the limiting reagent?
- Which reagent (N2 or H2) is the excess reagent?
- How many molecules of the excess reagent will be left?
- How many molecules of NH3 will form?

| N2 molecules |
H2 molecules |
NH3 molecules |
|
| Before the Reaction |
5 |
9 |
0 |
After the Reaction |
2 |
0 |
6 |
The answers to the questions above are:
- H2 is the limiting reagent
- N2 is the excess reagent
- 2 molecules of N2 are remaining
- 6 molecules of NH3 form
Limiting and excess reagent problems are among the most challenging in beginning chemistry. Make sure to review problems several times, ask questions, work hard, do not skip steps, and do not get frustrated!!
Last modified: Wednesday, August 4, 2010, 8:46 PM