Percent Yield and Chemistry
Percent Yield relates how much is actually accomplished to how much could have been accomplished in theory. For example, in 1000 at bats:
Ted Williams actually gets a hit only 344 times.
In theory, Ted Williams could have gotten a hit 1000 times.
His percent yield is (344/1000) = 0.344
0.344 is the Fraction Form of percent yield. Multiply by 100 to get it into a percent form:
(344/1000 * 100) = 34.4 %
Percent Yield is given by the following formula:
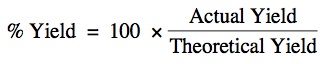
All of the mol - mol and mass - mass problems that we have performed so far ARE THEORETICAL PROBLEMS!!! The tell you the MAXIMUM POSSIBLE YIELD if you were to carry out the reaction.
Rarely does some one get 100% yield out of a reaction. % YIELD IS ALMOST ALWAYS LESS THAN 100%!!!
The actual yield is what result someone gets when they perform the reaction in the laboratory. It is the amount that they measure. ACTUAL YIELD SHOULD NEVER BE GREATER THAN THE THEORETICAL YIELD!!!
Summary:
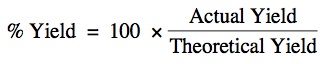
Actual Yield- The amount of product that actually forms when someone measures it in the laboratory.
Theoretical Yield- The maximum amount of product that can be performed as calculated by stoichiometry.
Intro Video:
Percent Yield relates how much is actually accomplished to how much could have been accomplished in theory. For example, in 1000 at bats:
Ted Williams actually gets a hit only 344 times.
In theory, Ted Williams could have gotten a hit 1000 times.
His percent yield is (344/1000) = 0.344
0.344 is the Fraction Form of percent yield. Multiply by 100 to get it into a percent form:
(344/1000 * 100) = 34.4 %
Percent Yield is given by the following formula:
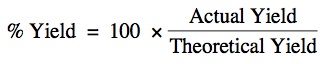
All of the mol - mol and mass - mass problems that we have performed so far ARE THEORETICAL PROBLEMS!!! The tell you the MAXIMUM POSSIBLE YIELD if you were to carry out the reaction.
Rarely does some one get 100% yield out of a reaction. % YIELD IS ALMOST ALWAYS LESS THAN 100%!!!
The actual yield is what result someone gets when they perform the reaction in the laboratory. It is the amount that they measure. ACTUAL YIELD SHOULD NEVER BE GREATER THAN THE THEORETICAL YIELD!!!
Summary:
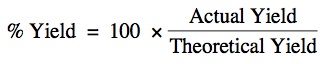
Actual Yield- The amount of product that actually forms when someone measures it in the laboratory.
Theoretical Yield- The maximum amount of product that can be performed as calculated by stoichiometry.
Intro Video:
Last modified: Thursday, August 12, 2010, 3:01 PM