The Limiting Reagent
Note: for the purposes of this unit the words "reactant" and "reagent" will be used interchangeably. They mean the EXACT SAME THING!!
Consider the following reaction:
N2 + 3 H2 ==> 2 NH3
Recall:
Answer: All of the N2 and H2 molecules would react producing 10 NH3 molecules (as shown in the picture below).
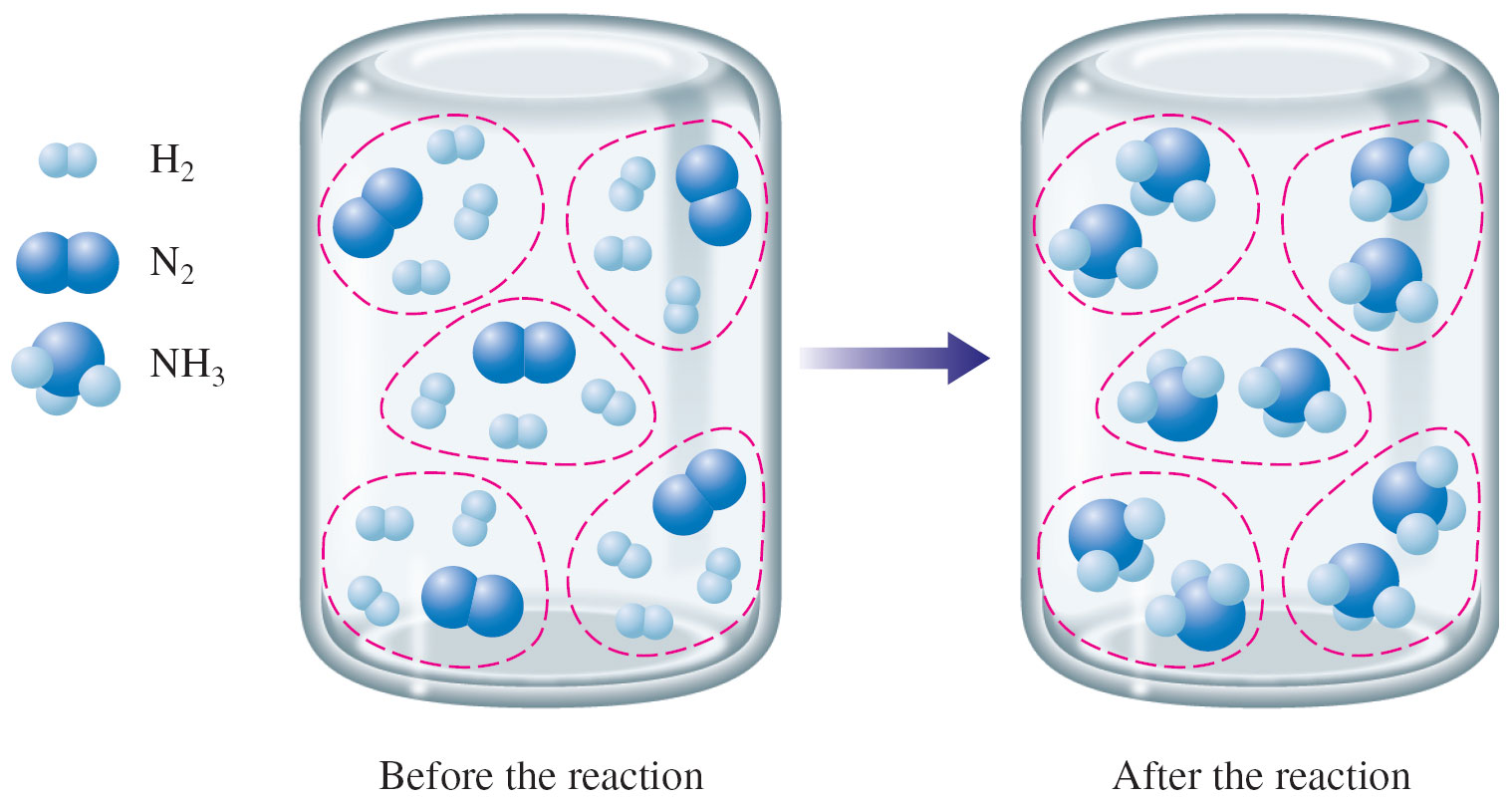
Notice that all of the N2 and all of the H2 is converted to NH3 in this example. Both reactants (reagents) being consumed entirely is extremely uncommon. Usually one runs out (which causes the reaction to stop) and the other reactant is left over.
Note: for the purposes of this unit the words "reactant" and "reagent" will be used interchangeably. They mean the EXACT SAME THING!!
Consider the following reaction:
N2 + 3 H2 ==> 2 NH3
Recall:
- The ratio of N2 to H2 is 1:3
- This ratio means that for every 1 N2 mole(cule) reacted, 3 H2 mole(cules) react
- For every 1 N2 mole(cule) that reacts, 2 NH3 mole(cules) are produced
- For every 3 H2 mole(cules) that react, 2 NH3 mole(cules) are produced
Answer: All of the N2 and H2 molecules would react producing 10 NH3 molecules (as shown in the picture below).

Notice that all of the N2 and all of the H2 is converted to NH3 in this example. Both reactants (reagents) being consumed entirely is extremely uncommon. Usually one runs out (which causes the reaction to stop) and the other reactant is left over.
- Limiting Reagent - The reagent (reactant) which is used up entirely in a reaction
- Excess Reagent - The reagent (reactant) which is left over after a reaction has stopped.
Last modified: Wednesday, August 4, 2010, 8:46 PM